- Polytech
- EMNS
-
Share this page
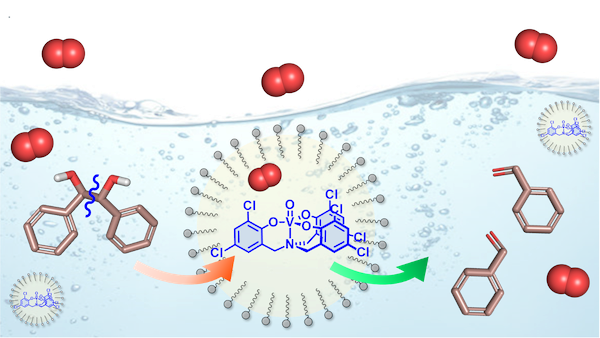
New publication: Vanadium Catalyst in Micelles: Towards a Greener Aerobic Oxidative Cleavage of Vicinal Diols in Water
Published on May 31, 2023
– Updated on June 2, 2023
Romain Carpentier, William Denis, Fatima Sanz Azcona, Davide Carraro, Glenn Grauwels, Manuel Orlandi, Cristiano Zonta, Giulia Licini and Kristin Bartik. ACS Sustainable Chemistry & Engineering 2023. First published: 01 June 2023
https://doi.org/10.1021/acssuschemeng.3c01820
Abstract
Aqueous micellar media offer an alternative to organic solvents for the development of safe and environmentally benign synthesis. A vanadium aminotriphenolate complex, known to be an effective catalyst for the aerobic oxidative C−C cleavage of vicinal diols in organic solvents, was incorporated in zwitterionic dodecyl phosphocholine (DPC) and in mixed TPGS-750-M:DPC micelles. It efficiently performed affording the corresponding carbonyl derivatives with no overoxidation, with catalyst loading down to 0.2 mol % and reaching TONs up to 500. The mixed micelles, which combine the enhanced stability conferred by zwitterionic DPC and the exceptional extraction properties of non-ionic TPGS-750-M, could be recycled and full conversion achieved upon reloading with catalyst and substrate. The study of the correlation between the reactivity of different substrates and their local concentration in the micellar core, derived from DOSY experiments, highlights that, while lipophilicity is important, the ease with which the substrate can access the catalyst, and therefore the location of the catalyst in the micellar phase, must also be considered when trying to rationalize reactivity in micelles.