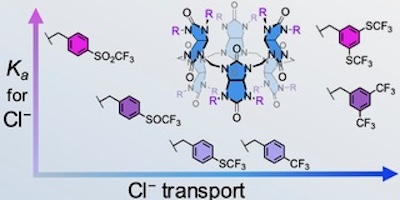
Eight different types of fluorinated groups were used to functionalise benzylated bambusuril macrocycles, resulting in a library of eleven anion receptors with high chloride affinities and very different anion transport rates.
Substituent effects of fluorinated bambusurils on their anion transport
By Matúš Chvojka, Vladimír Šindelář and Hennie Valkenier - First published: 10 April 2025
DOI: https://doi.org/10.1039/D5OB00400D
Abstract
Anionophores are molecules that can transport ions across membranes. Several structural design criteria must be met for anionophores to be highly active. Fluorinated anionophores are usually more potent than their non-fluorinated analogues due to their higher lipophilicity and increased affinity for anions. Clear structure–activity relationships have been described for small and relatively simple anionophores. However, such studies are more challenging for large and macrocyclic anionophores, as their preparation is usually more complicated, limiting the number of compounds tested in anion transport studies. Here we present a series of twelve macrocyclic bambusuril anion transporters to investigate how variations in fluorinated substituents affect their transport properties. Measurements of Cl−/HCO3− antiport activities in liposomes revealed links between parameters such as lipophilicity or substituent polarity and transport activity. For some bambusurils, an unusually large effect of the presence of cholesterol in the membrane on transport activity was found. Further studies showed that for very potent anion receptors, such as the bambusurils described here, the binding selectivity towards anions becomes more important than the absolute binding affinity to anions when considering anion exchange across the membrane.