- Polytech
- EMNS
-
Share this page
New paper on the synthesis of fluorinated bambusurils and their binding properties
Published on November 26, 2024
– Updated on November 26, 2024
By Matus Chvojka, Hennie Valkenier & Vladimir Sindelar - First published: 29 October 2024
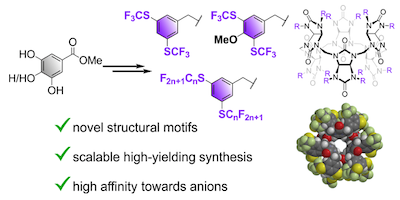
Synthesis of bambusurils with perfluoroalkylthiobenzyl groups as highly potent halide receptors
DOI: https://doi.org/10.1039/D4QO01746C
The preparation of anion receptors with ultrahigh binding affinities is an important, yet challenging, topic of supramolecular chemistry. The search for new structural motifs which would enhance the performance of anion receptors is therefore an important task. In this context, we report the synthesis of novel fluorinated bambus[6]urils that incorporate unique benzyl substituents with perfluoroalkylthio groups, aimed at enhancing their anion receptor capabilities. The synthetic strategy developed allows for the efficient preparation of these structural motifs. Ultrahigh stability of the complexes between halides and the prepared bambus[6]urils was observed and quantified using 19F NMR competition experiments. Replacing –CF3 groups on benzylated bambus[6]urils by –SCF3 groups increased the affinity of the macrocycles towards anions and provided the strongest iodide receptor reported with a binding affinity of 4 × 1013 M−1 in acetonitrile.