Effect of Surface Modification of Gold Nanoparticles Loaded with Small Nucleic Acid Sequences on Cytotoxicity and Uptake: A Comparative Study In Vitro. By Thuy Truong An Nguyen, Raphaël Dutour, Louise Conrard, Marjorie Vermeersch, Manon Mirgaux, David Perez-Morga, Nicolas Baeyens, Gilles Bruylants and Isabelle Demeestere - First published: 16 Mars 2025
Effect of Surface Modification of Gold Nanoparticles Loaded with Small Nucleic Acid Sequences on Cytotoxicity and Uptake: A Comparative Study In Vitro
https://doi.org/10.1021/acsabm.4c01861Abstract
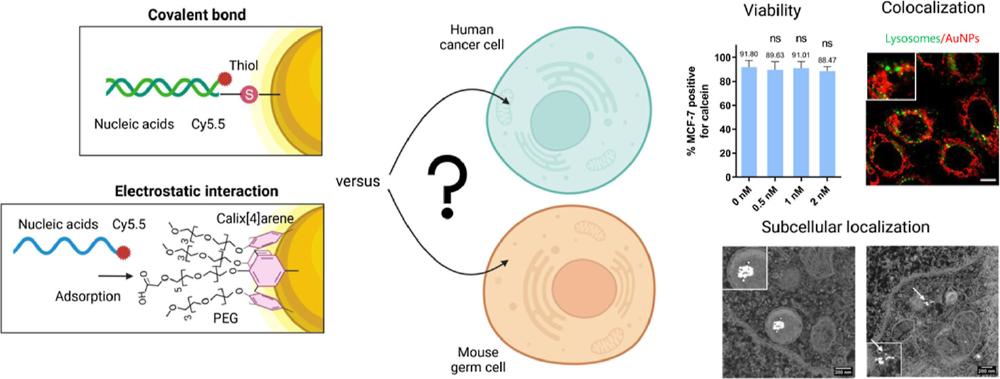
Nanoparticle technology, particularly gold nanoparticles (AuNPs), is being developed for a wide range of applications, including as a delivery system of peptides or nucleic acids (NA). Their use in precision medicine requires detailed engineering of NP functionalization to optimize their function and minimize off-target toxicity. Two main routes can be found in the literature for the attachment of NA strands to AuNPs: covalent binding via a thiol group or passive adsorption onto a specially adapted coating previously applied to the metallic core. In this latter case, the coating is often a positively charged polymer, as polyethylenimine, which due to its high positive charge can induce cytotoxicity. Here, we investigated an innovative strategy based on the initial coating of the particles using calix[4]arene macrocycles bearing polyethylene glycol chains as an interesting alternative to polyethylenimine for NA adsorption. Because any molecular modification of AuNPs may affect the cytotoxicity and cellular uptake, we compared the behavior of these AuNPs to that of particles obtained via a classical thiol covalent attachment in MCF-7 and GC-1 spg cell lines. We showed a high biocompatibility of both AuNPs-NA internalized in vitro. The difference in subcellular localization of both AuNPs-NA in MCF-7 cells compared to GC-1 spg cells suggests that their subcellular target is cell- and coating-dependent. This finding provides valuable insights for developing alternative NA delivery systems with a high degree of tunability.