Published on September 26, 2025
– Updated on September 26, 2025
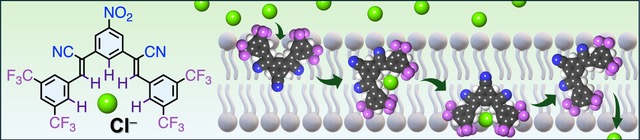
The joint project from Anurag and Lau on small chloride transporters has been published in Chemistry, A European Journal. These cyanostilbenes can bind chloride anions via CH-anion interactions and function as selective chloride transporters.
Cyanostilbenes as Selective Chloride Carriers Relying on CH-anion Interactions
by Dr. Anurag Singh, Dr. Lau Halgreen, Priyanka Rani Panda, Dr. Aaron Torres-Huerta, Dr. Nikolay Tumanov, Prof. Johan Wouters, Dr. Hennie Valkenier
First published: 19 August 2025
http://dx.doi.org/10.1002/chem.202502050
Abstract
Achieving selectivity is one of the challenges in developing anion receptors for transmembrane transport. This can be achieved through larger molecular designs that encapsulate the anion efficiently or by using alternative binding motifs instead of the most commonly used NH-based hydrogen bond donors. In this study, we present a series of small chloride receptors based on α-cyanostilbenes that use CH-based hydrogen bonds. By varying the substituents, we discovered that the compound containing four trifluoromethyl groups and a nitro group in addition to the two cyano groups exhibited the greatest affinity for chloride and the fastest chloride transport rates, surpassing the performance of reported triazole-based compound. Furthermore, this compound showed clear selectivity for transporting chloride over hydroxide, bicarbonate, and fluoride anions. The activity, selectivity, and deliverability of this compound make it a promising candidate for the treatment of chloride channelopathies.