In the same section
-
Share this page
Molecular recognition: from organic solvents to water

Molecular receptors capable of binding neutral or charged guests with a high affinity and selectivity have potential applications in fields ranging from biological and environmental monitoring to separation science and drug delivery.
A notable challenge is the development of receptors that work in an aqueous medium, where most biological and environmental processes occur. Water is indeed a competitive solvent. It screens polar interactions and as water molecules are excellent H-bond donors/acceptors they can efficiently solvate the different binding partners and actively interfere in the recognition processes.
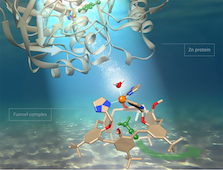
We undertake extensive physicochemical characterization (UV-Vis absorption and emission spectroscopies, microcalorimetry, advanced NMR methodologies) in order to elucidate the underlying factors that govern the molecular recognition processes.
Head: Prof. Kristin Bartik
Co-workers/researchers:
Collaborations: Prof. Ivan Jabin (ULB, Laboratory of Organic Chemistry), Prof. Benoit Colasson (Paris Descartes), Prof. Laura Rodríguez Raurell (University of Barcelona), Prof. Giulia Licini (University of Padova), Prof. Agnieszka Szumna (Polish Academy of Science)
Funding: COST, FNRS-FRIA PhD Fellowships
Publications:
Binding of Bioactive Ammonium Ions in Water with a Cavity-Based Selectivity: Water Solubilization versus Micellar Incorporation
Carpentier, R., Testa, C., Pappalardo, A., Jabin, I., & Bartik, K., JOC 90(1), 682–690 (2024).
Development of a water-soluble ouroboros-like calix[6]arene-trisimidazole-based ligand for enhanced binding of zinc
Carpentier, R., Lavendomme, R., Colasson, B., Bartik, K., & Jabin, I., Dalton transactions 54, 1052-1062 (2024).
Development of a Cone Homooxacalix[3]arene-Based Fluorescent Chemosensor for the Selective Detection of Biogenic Ammonium Ions in Protic Solvents
Lambert, S., Carpentier, R., Lepeintre, M., Testa, C., Pappalardo, A., Bartik, K., & Jabin, I., JOC 89(15), 10903–10911(2024).
Vanadium Catalyst in Micelles: Towards a Greener Aerobic Oxidative Cleavage of Vicinal Diols in Water
Carpentier R., Denis W., Sanz Azcona F., Carraro D., Grauwels G., Orlandi M., Zonta C., Licini G. & Bartik K., ACS Sustainable Chemistry & Engineering 11(23), 8633-8641(2023).
Supramolecular protection with a recyclable molecular container: an efficient strategy for the one-pot selective functionalization of polyfunctional substrates
Lambert S., Bartik K. & Jabin I., Org. Chem. Front. (2023).
Specific Binding of Primary Ammoniums in Aqueous Media by Homooxacalixarenes Incorporated into Micelles
Carpentier R., Lambert S., Brunetti E., Jabin I. and Bartik K., J. Org. Chem. 87 (19), 12749–12758 (2022).
A Water Molecule Triggers Guest Exchange at a Mono-Zinc Centre Confined in a Biomimetic Calixarene Pocket: a Model for Understanding Ligand Stability in Zn Proteins
Brunetti E., Marcelis L., Zhurkin F.E., Lhumer M., Jabin I., Reinaud O. & Bartik K., Chem. Eur. J. 27(55), 13730-13738 (2021).
Specific Binding of Primary Ammonium Ions and Lysine-Containing Peptides in Protic Solvents by Hexahomotrioxacalix[3]arenes
Lambert S., Bartik K. & Jabin I., The Journal of Organic Chemistry 85, 10062-10071 (2020).
Submerging a Biomimetic Metallo‐Receptor in Water for Molecular Recognition: Micellar Incorporation or Water Solubilization? A Case Study
Collin S., Parrot A., Marcelis L., Brunetti E., Jabin I., Bruylants G., Bartik K. and Reinaud O., Chem. Eur. J. 24, 17964-17974 (2018). ! Cover Picture !
Efficient Vanadium‐Catalyzed Aerobic C‐C Bond Oxidative Cleavage of Vicinal Diols
Amadio E., Gonzalez-Fabra J., Carraro D., Denis W., Gyoka B., Zonta C., Bartik K., Cavani F., Solmi S., Bo C. and Licini G., Adv. Synth. Catal. 360(17), 3286-3296 (2018).
A Selective Calix[6]arenes-based Fluorescent Chemosensor for Phosphatidylcholine Type Lipids
Brunetti E., Moerkerke S., Wouters J., Bartik K. and Jabin I., Organic and Biomolecular Chemistry 14, 10201-10207 (2016).
Colorimetric and fluorescence “turn-on” recognition of fluoride by a maleonitrile-based uranyl salen-complex
Bartocci S., Sabaté F.,Bosque R., Keymeulen F., Bartik K., Rodríguez L., Dalla Cort A., Dyes and Pigments 135, 94-101 (2016).
Primary Amine Recognition in Water by a Calix[6]aza-cryptand Incorporated in Dodecylphosphocholine Micelles
Brunetti E., Inthasot A., Keymeulen F., Reinaud O., Jabin I. and Bartik K., Organic & Biomolecular Chemistry 13, 2931-2938 (2015).
Fluoride Binding in Water with the Use of Micellar Nanodevices Based on Salophen Complexes
Keymeulen F., De Bernardin P., Giannicchi I., Galantini L., Bartik K. and Dalla Cort A., Organic & Biomolecular Chemistry 13, 2437-2443 (2015).
Fluorescent Chemosensors for Anions and Contact Ion Pairs with a Cavity-Based Selectivity
Brunetti E., Picron J.-F., Flidrova K., Bruylants G., Bartik K. and Jabin I., Journal of Organic Chemistry 79(13), 6179-6188 (2014).
Paramagnetic Relaxation Enhancement Experiments: A Valuable Tool for the Characterization of Micellar Nanodevices
Keymeulen F., De Bernardin P., Dalla Cort A. and Bartik K., J. Phys. Chem. B 117(39), 11654-11659 (2013).
Fluoride Binding in Water: A New Environment for a Known Receptor
Cametti M., Dalla Cort A. & Bartik K., Chem.Phys.Chem. 9, 2168-2171 (2008).
